Contents
- Target Outcome
- Prerequisites
-
Protocols
- Specimen Storage
- Specimen Thawing
- Specimen Dissection
- Patella Registration and Optotrak Marker Alignment
- Optotrak Base Plug Placement on Bones
- Registration Marker Placement on Femur and Tibia
- Optotrak Marker Assembly on Bones
- Acquisition of Anatomical Landmark Locations
- Acquisition of Registration Marker Locations
- Preparation of Specimen for Anatomical Imaging
- Preparation of Specimen for Mechanical Joint Testing
- Preparation of Specimen for Mechanical Tissue Testing
- Data Storage
- Data Dissemination
Target Outcome
- Overall readiness of the specimen to start experimentation
- Readiness of the specimen for registration
- Readiness of the specimen for anatomical imaging
- Readiness of the specimen for joint mechanics testing
- Readiness of the specimen for tissue mechanics testing
Prerequisites
Infrastructure
Previous Protocols
Related Protocols
Protocols
Specimen Storage
- Specimen Storage
- Storage Location
- Specimens should be stored in the walk-in freezer on the third floor in room ND3-77B
- Serological testing sheets should be scanned and saved in the repository directory upon delivery
- A log should be filled out on the data sheet found in ND3-77B with:
- Specimen number
- PI name being Ahmet Erdemir
- Shelf where the leg was placed labeled accordingly
- Source: Human
- Serological testing sheets for each specimen should be accounted for and should remain in room ND3-77B so long as a specimen still remains in the freezer.
- Specimen Packaging
- Specimens can be directly placed in the freezer in their original packaging after being procured from the company
- Specimens should be packaged as followed after handling it:
- Wrap specimen in a blue chuck (found in cabinet in room ND1-04).
- Specimen chuck should be labeled with the specimen number, the side (right or left) and what it is (knee)
- The wrapped specimen should be placed in a red biohazard bag found in the same cabinet in ND1-04 as the chucks and labeled in the same fashion as the chuck.
- Storage Location
Specimen Thawing
- Specimen thawing
The specimen should be taken to the BioRobotics Cadaver Lab in room ND1-04 and thawed for 12-18 hours (potentially up to 24 hours) prior to specimen preparation at room temperature.
- The specimen should be unwrapped in order to thaw in less than 24 hours.
- For thawing periods greater than 24 hours (i.e. through the weekend) leave the specimen wrapped.
Specimen Dissection
- Specimen dissection:
- Mark proximal and distal regions for tissue removal
- Femur: 4.5 inches proximal, measured from the epicondylar axis
- Tibia: 4.5 inches distal, measured from the epicondylar axis
- Remove all tissue proximal to thigh mark to expose hip.
- Isolate the quadriceps tendon by removing tissue around it and keep it hydrated by wrapping it in saline soaked towel. Make sure to leave adequate amount of quadriceps tendon for clamping.
- Remove a four inch wide strip of tissue distal to tibia mark to expose tibia and fibula shafts. All the tissue on the tibia and fibula shafts should be removed. The tibia should be exposed as proximal as possible without damaging joint capsule to allow room for plugs (see below).
The rest of the soft-tissue will remain throughout testing due to possible effects peripheral soft-tissue has on joint response as described in Peripheral_Soft-Tissue_Effects.pdf
- Mark proximal and distal regions for tissue removal
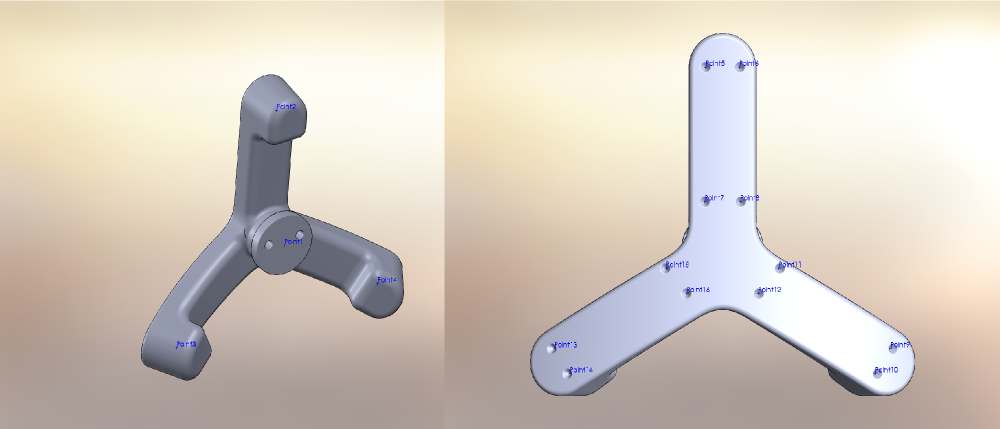
Patella Registration and Optotrak Marker Alignment
- Collect data to associate Optotrak and registration marker coordinate systems for patella: This can be done in parallel with specimen dissection or before/after the whole experiment is done.
- Place an Optotrak marker on a piece of wood.
- Place patella base plug on the same piece of wood.
- Place patella Optotrak marker on patella base plug.
- Secure the wood (not needed but good).
- Make sure Optotrak system is calibrated and ready to roll.
- Record location of Optotrak markers (wood + patella base plug) (these are measured in global Optotrak coordinate system)
- Replace patella Optotrak marker with patella registration marker assembly on patella base plug.
- Record location of Optotrak marker on wood and patella registration marker assembly divots with Optotrak probing device in the order illustrated below.
- Remove base plug from wood.
- Now, we can establish the transformation matrix between patella registration marker coordinate system and patella Optotrak marker coordinate system.
Optotrak Base Plug Placement on Bones
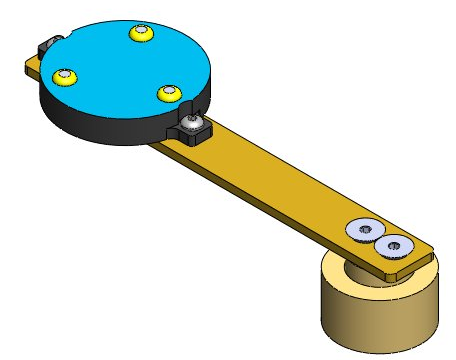
- Base plug placement
The use of an optoelectronic camera system (Optotrak, Northern Digital Inc., Waterloo, Ontario, Canada) requires repeatable placement of the orthopedic research pin markers http://www.ndigital.com/lifesciences/spineresearchpins.php
- In order to ensure repeatable placement, three plastic (MRI compatible) fixtures were designed to be rigidly secured to the tibia, femur, and patella throughout experimentation.
The fixtures and markers can be found in the labeled shelves in the BioRobotics Cadaver Lab.
- The fixtures are composed of:
- A threaded base plug attached to the bone through the use of three brass screws. Bone cement is used to ensure firm connection between the screws and bone. For experiments requiring MRI imaging, polymer based plugs should be used. If MRI imaging is not required, brass plugs are permissible.
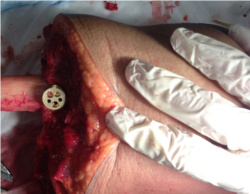
- A second brass fixture (for motion tracking) can be securely screwed onto the plug through the used of two pins in a repeatable fashion. This brass fixture will hold the Optotrak markers for the tibia and femur (see figure below). For patella it will hold the Optotrak markers OR registration marker assembly.
- The fixtures mounted on the bones with caution to the following issues:
- Three screws (#3) will secure each fixture to the bone.
- Holes should be drilled on the bone to secure the screws, using fixture as a template. Use drill bit diameter ~.088 in; tapping is not needed. Drill length should be short to ensure patella cartilage is not compromised.
- Before screwing in, use a k-wire in one hole to hold plug still when screwing in others.
- To ensure the screws securing the base plugs to the bone don't loosen.
- Make small batches of bone cement (one plug at a time) to avoid cement hardening (clumped up cement is not good; make a new batch). Bone cement should be mixed in the fume hood and masks should be worn.
- Dip the screw in the bone cement; wipe off excess cement from screw before inserting.
- Screw into the bone prior to hardening.
- If a screw shears off, continue as long as 2 screws are fully engaged; leave the sheared one in plug.
- If all screws shear then remove screws and try drilling bigger holes first. You may want to test with non-cemented screws first to see if they shear.
- Femur - ~4 inches from the epicondylar axis
- The fixture should be mounted on the medial side of the femur and the pin holes should be aligned parallel to the femoral shaft with the two screw holes oriented on the anterior side and the single screw hole oriented on the posterior side (brass base plug shown below)
- The fixture should be mounted on the medial side of the femur and the pin holes should be aligned parallel to the femoral shaft with the two screw holes oriented on the anterior side and the single screw hole oriented on the posterior side (brass base plug shown below)
- Tibia - ~3 inches from the epicondylar axis
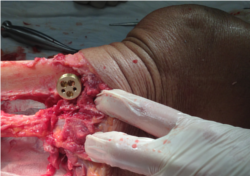
- The fixture should be mounted on the lateral side of the tibia and the pin holes should be aligned parallel to the tibial shaft with the two screw holes oriented on the anterior side and the single screw hole oriented on the posterior side (brass base plug shown below)
- The fixture should be mounted on the lateral side of the tibia and the pin holes should be aligned parallel to the tibial shaft with the two screw holes oriented on the anterior side and the single screw hole oriented on the posterior side (brass base plug shown below)
- Patella - on the center of the patella
- Expose the bone by cutting the skin on a vertical line and retract to the sides to make room for the plug.
- The fixture should be mounted on the anterior side of the patella tibia and the pin holes should be aligned such that the two screw holes are on the inferior side.
Registration Marker Placement on Femur and Tibia
- Placement of registration markers
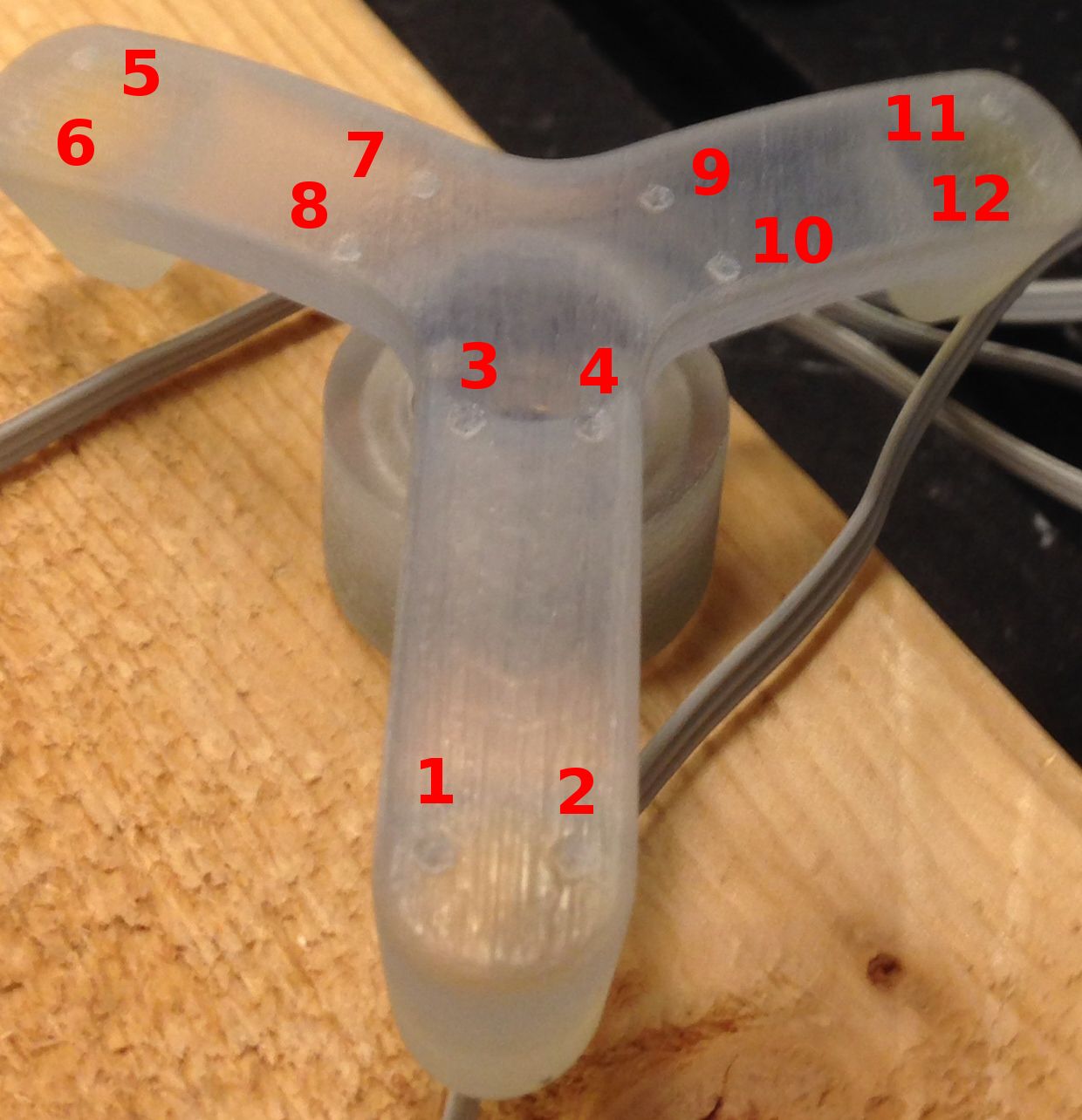
- Three spherical markers should be rigidly mounted to the tibia and the femur as close to joint line as possible without damaging ligamentous structures (~2 to 2.5 inches). The volume of placement is simply driven by the field of view constraints of anatomical imaging.
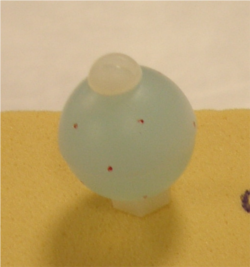
- Spherical markers are hollow plastic spheres (10 mm radius ) filled with water based gel and are attached to the bones using plastic screws (#8-32) for the tibia and femur for MRI registration. The screw axis and sphere centers should be concentric. If not, careful handling is necessary to prevent rotation of the markers after their installation on the bone.
- Spherical markers are hollow plastic spheres (10 mm radius ) filled with water based gel and are attached to the bones using plastic screws (#8-32) for the tibia and femur for MRI registration. The screw axis and sphere centers should be concentric. If not, careful handling is necessary to prevent rotation of the markers after their installation on the bone.
- Tissue may need to dissected to make room for the markers.
- Holes to place the registration markers should be drilled on the bone using drill bit size of 0.136 in. Holes should be tapped for #8-32 using a drill wire gage 29.
- Femur spheres should be mounted on the anterior or anteriomedial, medial, lateral sides of the bone. An alternative marker assembly is medial, lateral, and posterior. This helps when the quadriceps tendon does not allow placement of an anterior/anteriomedial registration marker.
- Tibia spheres should be mounted on the medial, lateral, posterior sides of the bone.
- Patella registration markers are not needed at this point as their association to patella Optotrak markers should be obtained as described above.
- Three spherical markers should be rigidly mounted to the tibia and the femur as close to joint line as possible without damaging ligamentous structures (~2 to 2.5 inches). The volume of placement is simply driven by the field of view constraints of anatomical imaging.
Optotrak Marker Assembly on Bones
- Mounting of Optotrak markers:
- Place the femur Optotrak marker on femur base plug
- Place the tibia Optotrak marker on tibia base plug
- The tibia Optotrak marker is a rigid assembly consisting of three normal Optotrak markers, allowing for a larger Optotrak measurement view
- The tibia Optotrak marker is a rigid assembly consisting of three normal Optotrak markers, allowing for a larger Optotrak measurement view
- Place the patella Optotrak marker on patella base plug
Acquisition of Anatomical Landmark Locations
- Anatomical landmark data collection:
- Move specimen to Optotrak measurement view.
- Make sure Optotrak system is previously calibrated and ready to roll
- Use the digitizing probe to record anatomical locations along with Optotrak marker position/orientation output, measured with respect to the global Optotrak coordinate system for each respective bone
- The following anatomical locations should be recorded:
- Femur landmarks
- F1. Lateral epicondyle of the femur (most lateral point)
- F2. Medial epicondyle of the femur (most medial point)
- F3-F6. 4 points around the epiphyseal line of the femur
- Tibia landmarks
- T1. Medial tibial plateau (most medial point)
- T2. Lateral tibial plateau (most lateral point)
- T3. Medial malleolus of the tibia (most medial point)
- T4. Lateral malleolus of the fibula (most lateral point)
- Patella landmarks
- P1. Most lateral point
- P2. Most medial point
- P3. Most superior point
- P4. Most inferior point
- Femur landmarks
- Now, we can describe the anatomical points in their respective Optotrak body coordinate systems.
Acquisition of Registration Marker Locations
- Registration marker data collection
- Keep specimen within Optotrak measurement view.
- Use the digitizing probe to record registration marker locations along with Optotrak marker position/orientation output, measured with respect to the global Optotrak coordinate system for each respective bone
- Ten points on each spherical marker should be digitized such that they are distributed evenly about the sphere surface
- The spheres should be digitized in the following order:
- Femur: anterior, medial, lateral markers (medial, lateral, posterior if using alternative marker placement; see registration marker placement above)
- Tibia: lateral, medial, posterior markers
- Now, we can describe registration markers in their respective Optotrak body coordinate systems.
Preparation of Specimen for Anatomical Imaging
- Prepare specimen for MRI:
- Remove Optotrak markers from their respective base plugs on femur, tibia, and patella
- Cut bones
Use a hack saw found in labeled cabinet in the BioRobotics Cadaver Lab
- Clamp and cut femur ~7.5 inches proximal to the epicondylar axis. File the end of the bones to prevent sharp edges.
- Clamp and cut tibia and fibula ~7.5 inches distal to the epicondylar axis. File the end of the bones to prevent sharp edges.
- Secure tibia and fibula to each other using a zip tie and a spacer.
- The foot should be preserved for future testing (following storage procedures described above)
- Place patella registration marker assembly on patella
Now, the specimen is ready to go through Specifications/ExperimentationAnatomicalImaging
Preparation of Specimen for Mechanical Joint Testing
- Prepare specimen for joint testing
- After specimen comes from MRI; the registration markers can be removed.
- Secure the fibula to the tibia by passing a drill bit (or a wood screw) through both bones and leaving it there. Try to keep relative positioning of the tibia and fibula anatomical.
- Potting
- Preparation
- Safety
- Gloves, face shields, gowns, protective eye-wear and welders gloves should be used at all times by people handling hot liquid metal
- Melting woods metal
The woods metal found in the hood of the BioRobotics Cadaver Lab should be melted.
- For quickly melting it, turn the temperature of the hotplate to 300 degrees until melted.
- Once melted:
- If the woods metal isn't already in the aluminum pitcher, CAREFULLY pour it into it and place the pitcher on the hotplate.
- Turn the temperature to 100 degrees.
Two aluminum tubes (3 inches long, 2.5 inch OD) can be found in the labeled cabinet in the BioRobotics laboratory.
Clay can be found in the BioRobotics laboratory in the first cabinet on the left side of the room. Approximately, a fist full should be taken.
- Safety
- Potting the tibia end.
- Before potting, check whether Optotrak markers will fit on plugs with the pot.
- Roll the clay flat so that the aluminum tube can sit on it and be completely sealed.
- Place the clay on the the dissection table on the blue side of a chuck.
- If there are drill-bit holes in the tube, orient the tube on the clay so the hole side is not touching the clay
- Seal the bottom of the tube completely with clay and plug any holes with bits of clay
- Place tibia in the tube and hold the extended knee in place
- Verify the length from the bottom of the tube to the epicondylar axis as ~7.5 inches
- Verify the center of the epicondylar axis is inline, vertically, with the bottom of the tube, from both sagittal and anterior views.
- Use a beaker to fill the tube with water and refill the beaker.
- A foil funnel found in the hood should be held over the top of the tube with forceps by the person holding the knee.
- A different person should CAREFULLY ladle the HOT woods metal into the funnel filling the tube completely.
- Cold water from the beaker can be poured on the tube several times in order to quickly solidify the woods metal.
- Approximately 5 minutes are needed for it to completely harden.
- Remove the clay from the bottom of the tube.
- Flip the knee so that the bottom of the tibia tube is pointed up.
- Top the tube off by CAREFULLY ladling hot woods metal into it.
- Pour cold water on top of it again and wait for it to harden.
- Using a chisel remove the excess wood metal that hardens over edge of the pot.
- Potting the femur end.
- Before potting, check whether Optotrak markers will fit on plugs with the pot.
- Roll the clay flat so that the second aluminum tube can sit on it and be completely sealed.
- Place the clay on the dissection table on the blue side of a chuck.
- If there are drill-bit holes in the tube, orient the tube on the clay so the hole side is not touching the clay
- Seal the bottom of the tube completely with clay and plug any holes with bits of clay.
- Place femur in the tube and hold the extended knee in place.
- Verify the length from the bottom of the tube to the epicondylar axis as ~7.5 inches
- Verify the center of both tubes are inline, vertically, from both sagittal and anterior views.
- Use a beaker to fill the tube with water and refill the beaker.
- A foil funnel found in the hood should be held over the top of the tube with forceps by the person holding the knee.
- A different person should CAREFULLY ladle the HOT woods metal into the funnel filling the tube completely.
- Cold water from the beaker can be poured on the tube several times in order to quickly solidify the woods metal.
- Approximately 5 minutes are needed to completely harden.
- Remove the clay from the bottom of the tube.
- Flip the knee so that the bottom of the femur tube is pointed up.
- Top the tube off by CAREFULLY ladling hot woods metal into it.
- Pour cold water on top of it again and wait for it to harden.
- Using a chisel remove the excess wood metal that hardens over edge of the pot.
- Preparation
- Secure potted specimen
- Two 3 inch long drill bits (~1/8 inches in diameter) will be driven in to each tube in order to prohibit motion between the bone and the pot.
- Holes in the tube should be utilized if possible.
- Each drill bit should be driven in so that it goes through both sides of the tube and through the bone.
- Attempts to drill through the part of the tube closest to the joint should be made.
- The two drill bits on each bone should NOT be oriented parallel to one another.
- Prepare extensor mechanism (for patellofemoral joint testing only)
- These activities may happen throughout the various specimen preparation stages listed above. They are provided in here to ensure their execution.
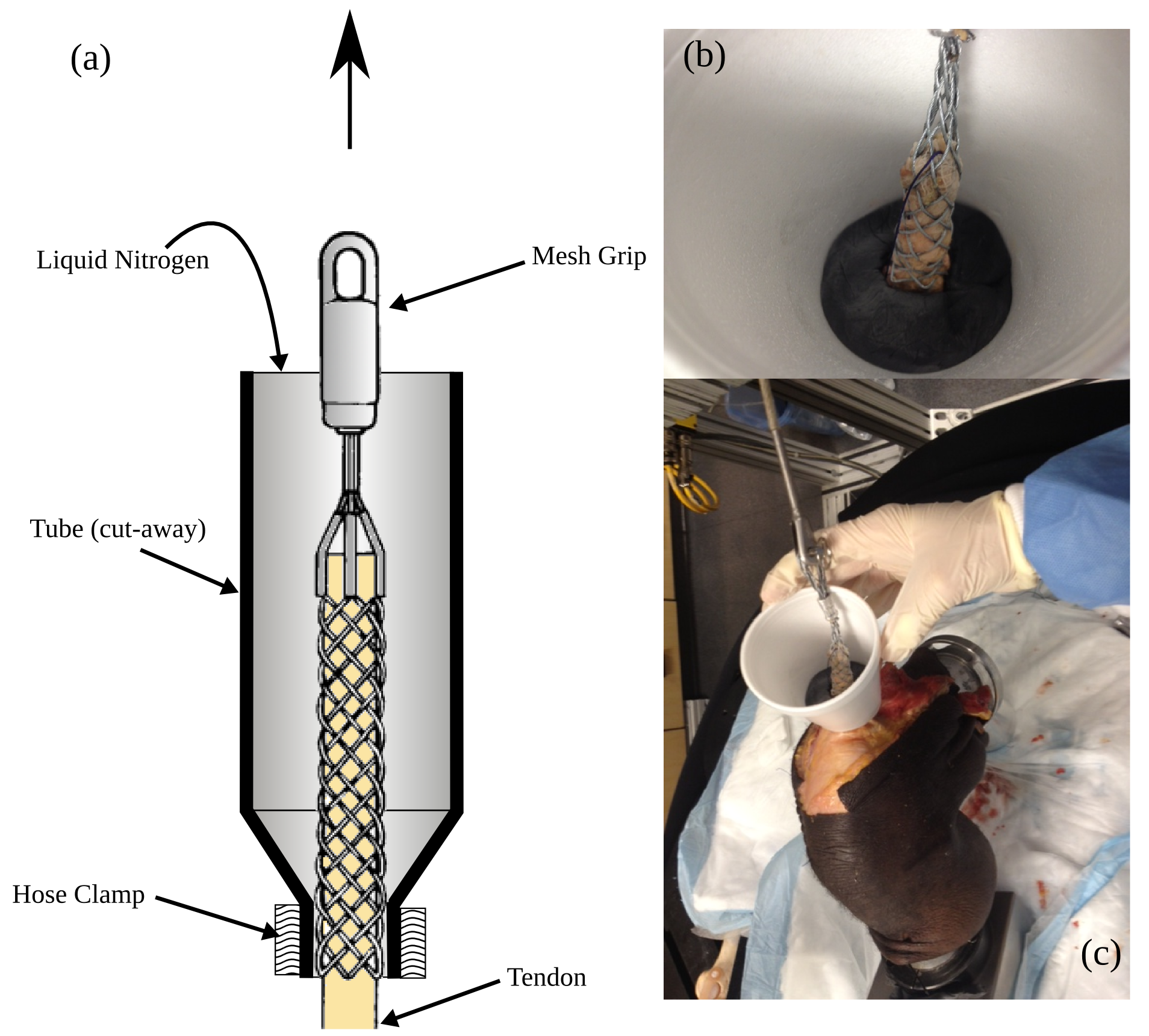
- Clamp quadriceps tendon (using liquid nitrogen)
Different sized cable-pulling grips can be found in the labeled cabinet in the BioRobotics laboratory.
- Example figure below shows a (a) schematic of the clamp setup, (b) a close-up view of the clamp and tendon in the nitrogen freezing tube and (c) a potted knee with a clear view of the quadriceps tendon clamp in place.
- The tendon should be carefully isolated as close to the joint center as possible.
- The clamp should be positioned such that its loop will be slightly above the bottom plane of femur pot. The tendon may need to be cut to accommodate this positioning.
- Suture can be used to consolidate the quadriceps tendon into a uniform and dense tubular structure using the whipstitch technique.
- Suture at the end of the tendon should be threaded through and out of the pulling end of the clamp.
- The clamp should then be compressed so the tendon can be fully inserted into it by pulling on the suture.
- Once fully in the clamp, applying tension to the clamp should fully encapsulate the tendon.
- Applying a manual load to the clamp should not cause the tendon to slip out
- If the tendon does slip out after applying a manual load, consider using a smaller diameter and/or longer clamp
- Tendon should be ready for freeze clamping during the experiment; when appropriate follow the steps below:
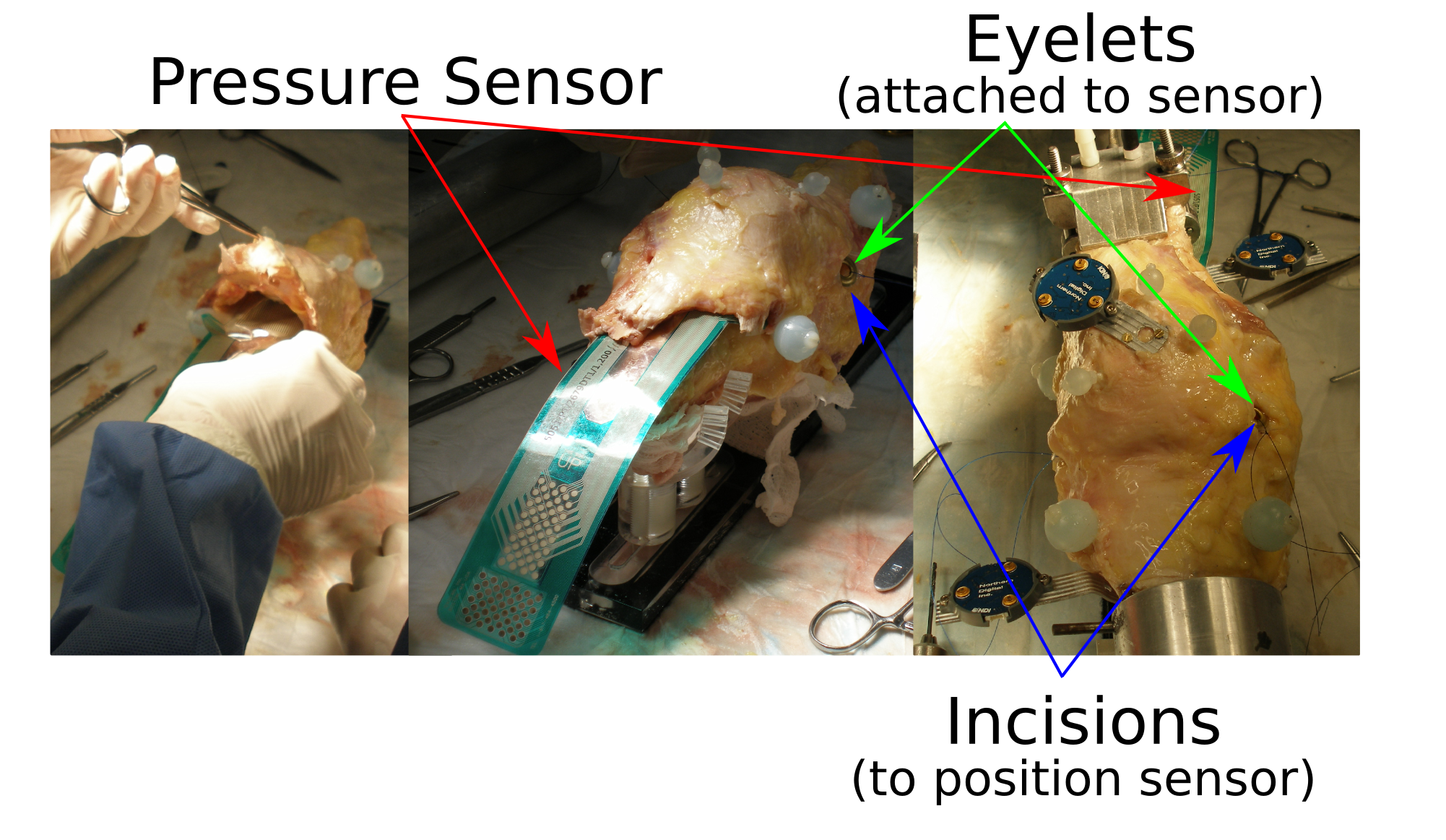
- Prepare quadriceps area for pressure sensor insertion (which can be conducted during the experimentation)
- Specific preparation for patellofemoral testing includes accommodating the pressure sensor. The sensor should be small enough to fit inside the patellofemoral joint but large enough to cover the contact surface.
Please refer to Specifications/PressureCalibration for specifics about preparing the sensor for testing.
- A space beneath the quadriceps tendon will have to be opened adequately to accommodate the pressure sensor (left in the above picture) Without disrupting the surrounding capsular structure, the sensor insertion area should be cleaned up to ensure that the sensor does not catch to any tissue structure during insertion.
- The sensor can be inserted in one of the following ways depending on the knee size:
- Insertion method for large knees and for better securing of the sensor in place:
- Vertical incisions, one medial to and the other lateral, will be made proximal to the tibial insertion of the patellar tendon. Space these wide enough to "feed" the bottom of the sensor through these holes. Make sure the incisions to not compromise any mechanically important features of the joint.
- Eyelets (grommets) will need to be assembled after assessing approximate location of the pressure sensor relative to the specimen. Tie suture threads are tied to these eyelets so the sensor can be easily positioned (and pulled taut) through the vertical incisions during testing.
- Insertion method for small knees and for quick placement of sensor:
- This method does not use any eyelets or suture threads to pull the sensor through vertical incisions. Vertical incisions are therefore not necessary.
- Curve the sensor transversely to give it some stiffness along the insertion direction. Push the sensor through the joint as far as possible, feeding it in the joint space with your fingers. The sensor may be placed when the specimen is on the robot.
- Insertion method for large knees and for better securing of the sensor in place:
- Place Optotrak marker assembly on patella, femur, and tibia base plugs.
Now, the specimen is ready to go through Specifications/ExperimentationJointMechanics
Preparation of Specimen for Mechanical Tissue Testing
Notes
- Photos should be taken at every step with appropriate labeling to facilitate cataloging.
Harvest secondary tissues (see list in Specifications/ExperimentationTissueMechanics) as feasible for testing at a later time.
Harvesting the tissues
- Once joint testing is completed, harvest all primary and secondary tissues.
- Dissections will be performed by surgeons but in case one of the team members has to perform it, follow directions below.
- Starting with an incision axially, remove as much of the skin, fat and muscle tissues as possible.
- Quadriceps tendon-patella-patellar ligament complex is exposed at this point. Carefully separate it from the joint. Here, care needs to be taken to preserve as much tissue from the tendon and ligament as possible.
- Once the joint space is exposed, work on the lateral collateral ligament and carefully harvest it by cutting closely at the insertion sites.
- At this point lateral meniscus will be exposed enough to be harvested intact. Make cuts as close to horn insertions as possible.
- Next harvest the medial collateral ligament-medial meniscus complex together as the meniscus is attached to the ligament and will be easier to separate once the combined structure is separated from the joint. Separate the mcl and medial meniscus.
- This will expose the acl and pcl which can be easily harvested once all the surrounding tissues are removed.
- Label all tissues using data labeling specifications. Mark tissue orientation for every tissue bag using arrows.
- As soon as the tissues are removed, the labeler should label the tissue clearly identifying the type and anatomical directions of tibia and femur insertion site ends, i.e., depiction of tibial and femoral ends, anatomical orientation of sides, e.g., medial or lateral, anterior or posterior.
- Place the tissues as harvested in a pre-labeled bag indicating the specimen #, name, and orientation.
- Naming convention can be found in data management specifications.
- Separate the tibia, femur, and patella with articular cartilage intact on all bone surfaces.
- Remove cartilage from medial and lateral femoral condyle, medial and lateral tibial plateau, patella and trochlear groove using a scalpel blade as close to the bone surface as possible. Here, as much tissue should be removed from each location as possible at one time.
- Place cartilage strips in a pre-labeled bags indicating the location and orientation.(Take pictures at every step to later associate every strip and its location, the orientation is particularly important to obtain tensile samples from the superficial zone and the fiber orientation needs to be clearly identified).
Tissue Preservation
If time constraints during tissue harvesting do not allow sample preparation (see below), the preserve the tissue samples carefully.
- Wrap each tissue separately in saline soaked gauze or paper towel and store in labeled bags.
- Place all the tissues harvested and bagged in a container, put the specimen number on the container before placing in the freezer.
- Keep the tissue container in a freezer connected to a backup generator.( A freezer is also available on the third floor of Department of Biomedical Engineering, Cleveland Clinic).
- When storing tissues in the freezer, fill the login sheet outside with the the shelf location, specimen label and date.
Test Sample Preparation
Note: Each sample is prepared the day it is to be tested. Ligament and tendon samples are taken frozen immediately to histochemistry lab to be cut with cryostat and tested the same day. If the testing cannot be done the same day, keep the sample in the fridge and make a note on the relevant wiki page.
Cartilage
Acquire 5 mm diameter full thickness cylindrical sample and 5mm by 1mm tensile sample (punch specifications can be found in the experimentation mechanics infrastructure page).
- For each cartilage strip, punch one tensile and one compression sample. Use same compression sample for both confined and unconfined compression tests.
- For tensile samples especially of femur and patella, use orientation of superficial fibers to align the tensile sample long axis.
- To punch out samples, place the harvested cartilage strips on the plate of the hand press located in ND1-06A in the Department of Biomedical Engineering, Cleveland Clinic
- Place a rubber sheet underneath the sample.
- Set the punch on top of the tissue over the region where the sample should be harvested.
- Lower the press completely until a sample separates from the harvested tissue strip.
- For compression test:
- To obtain uniform thickness compression samples, minimally shave the sample using a vibratome (Leica VT1200S) immediately prior to testing. (The vibratome is available in ND1-06A in the Department of Biomedical Engineering, Cleveland Clinic.)
- Apply tissue adhesive to the bottom of the cartilage sample and place on the vibratome detachable plate.
- Place the plate on the plate holder base of the vibratome.
- Attach the blade at an appropriate angle (carbon steel razor blades are preferable).
- Manually raise the plate until the sample and blade are aligned and the top of the sample is in the same plane as the blade.
- Adjust the amplitude and speed settings. These may vary depending on the sample.
- Slowly move the blade towards the sample.
- Remove 50 microns at a time until a flat surface is achieved (this assessment is done visually).
- Use a tissue debonder to detach the sample.
- Note: Use adhesive and debonder sparingly as the effects if these on the tissue properties are not established.
- For uniaxial tension:
- Obtain tensile samples from the articular surface. Glue the bottom of the punched full thickness sample to the vibratome plate and very carefully remove 400-500 micron thick sample.
- Note: The uniformity of this sample relies mainly on how uniformly thick the initial full thickness sample is. To preserve the articular surface and separate as much of the superficial zone for tensile test, first try to manually make the punched full thickness tensile sample fairly uniform. This can be done by placing the sample horizontally with the articular surface flush with some vertical surface and using a carbon steel blade manually cut the non-uniform bottom. If done carefully, this will yield a fairly uniform sample which then can be placed on the vibratome to extract the superficial layer for tensile test.
Meniscus
- Divide the meniscus sample into three regions (anterior, middle and posterior).
- For uniaxial tension:
- Sample dimensions are 5 X 2 mm (800 micron thickness) dumbbells.
- Obtain the tensile sample from the middle region such that the punch long axis aligns with the circumferential fiber direction.
- To obtain uniform thickness samples, first obtain layers/strips of meniscal tissue with desired thicknesses. This can be done using the vibratome. (The vibratome can be found in ND1-06A. All tools and accessories can be found in the top drawer below it.)
- Apply adhesive to the tibial side of the meniscus block and place on the plate.
- Place the plate on the magnet on the vibratome.
- Fix the blade onto the holder at an appropriate angle.
- Raise the plate manually until the sample and blade are aligned and the top of the sample is in the same plane as the blade.
- Adjust the amplitude and speed settings.
- Slowly move the blade towards the sample.
- Remove 50 microns at a time until a wide enough flat surface is achieved (enough to accommodate the punch).
- The tensile sample is obtained from the deep zone where the fibers are circumferentially oriented.
- Once flat surface is achieved that can accommodate the tensile sample, obtain 800 microns thick samples.
Multiple samples can be obtained and should be labeled by layer. For sample labeling, see Specifications/DataManagement
- Use the debonder to remove the leftover sample.
- Pick one or multiple (if needed) of these uniform thickness layers and obtain a tensile sample using the appropriate tensile punch.
- Place the sample on a rubber sheet.
- Place the punch on the tissue layer with the long axis of the punch aligned with the circumferential fibers.
- Lower the press completely until the dumbbell sample separates.
- For compression test:
- Sample dimensions are 5 mm diameter and full thickness.
- Uniform thickness samples for compression tests can be obtained from posterior or anterior blocks. Label the sample appropriately.
- To obtain a uniform thickness sample first get a uniform thickness block. This can be done using the vibratome (details of location can be found in meniscus tensile sample preparation above)
- Apply adhesive to the tibial side of the meniscus block and place it on the plate.
- Fix the blade on the holder of the vibratome.
- Manually raise the plate until the sample and blade are aligned and the top of the sample is in the same plane as the blade.
- Adjust the amplitude and speed settings.
- Slowly move the blade towards the sample .
- Remove 50 microns at a time until a flat surface is achieved that can accommodate the cylindrical punch.
- Use the debonder to remove the flat block from the plate.
- Place the block on a rubber sheet and place it on the press.
- Place the cylindrical punch on top of the block from where the sample needs to be harvested.
- Lower the press until the 5 mm diameter disk separates.
Ligaments and Tendon
- For uniaxial tension
- Sample dimensions: 10 X 2 mm (~1 mm thickness) dumbbells.
- MCL tensile sample can be obtained without thinning the tissue as it is a fairly thin ligament. To thin rest of the ligaments and tendon to desired dimensions, use cryostat.
- Currently to reserve the cryostat contact Ronald Midura, PhD until a technician is appointed at the Histochemistry Core in the Department of Biomedical Engineering.
- Do not thaw the samples before thinning on the cryostat.
- Remove the wrapped paper towel by holding the tissue under cool water briefly.
- Measure the tissue thickness with calipers (available in Histochemistry Core).
- If the ligament thickness is greater than 1 mm, use the cryostat to remove excess tissue.
- Place the tissue on the circular mounting disc using the OCT compound (both available in Histochemistry Core).
- Place the disc and sample in the cryostat for a few minutes so that the OCT compound freezes with the sample.
- Once the sample and OCT compound are frozen attach and lock the disc on the holder. Also lock the blade.
- Once the tissue and blade are secure, using the manual handle on the right side of the cryostat start shaving 50um at a time.
- The goal is to remove 1 mm on one side, flip the sample and remove 1 mm on the other side, until the sample thickness is close to 1mm.
- Once 1mm on one side is done, take the disc and sample out of the cryostat, place it on the heat sink, this will melt the OCT compound enough that sample can be detached still frozen. Measure the thickness after every time the sample is detached from the disc.
- Once the desired thickness sample is obtained, immerse the sample in saline and transport to the tissue testing lab for punching and testing.
- For punching the sample follow the instructions provided for cartilage or meniscus tensile samples. (Use 10 X 2 mm punch)
Data Storage
All data relevant to specimen preparation, e.g., anatomical landmark locations, registration marker positions, etc. should be uploaded to the in-house data management system (http://cobicore.lerner.ccf.org/midas, accessible only within the Cleveland Clinic network) for prospective organization (Open Knee(s) Community). Following organization, a documented version of the data will be disseminated at Open Knee(s) project site (https://simtk.org/home/openknee).
Data Dissemination
Data from this project is available for downloading.