|
Size: 8685
Comment:
|
Size: 8689
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 150: | Line 150: |
| ||||'''''Segment Coordinate system definition (Right and Left)''''' || | ||||||||'''''Segment Coordinate system definition (Right and Left)''''' || |
Contents
PART I: Indentation
See Specifications/InVitroTesting/PartII for Part II: Surgery
Logistics
Part 1 - Indentation |
|
Day 1 |
Day 2 |
Day 3 |
Morning |
Thaw Specimen |
Anthropometric/MRI Prep 8-9:30 |
Harvest Tissue |
Mid |
Thaw Specimen |
CT & MRI 10-12 |
Freeze |
Afternoon |
Thaw Specimen |
Ultrasound |
|
Target Outcome
- Demographic documentation (age, gender)
- Anthropometric measurements (body mass, height, extremity lengths and circumferences)
- A set of MR and CT images of upper and lower leg.
- Ultrasound images at unloaded state to extract tissue thickness for various extremity locations
- Ultrasound imaging during indentation to extract tissue thickness for various extremity locations
- Mechanical testing of tissue and tissue-interface to characterize mechanical properties at ultrasound indentation locations
Prerequisites
Specifications
Infrastructure
Protocols
Input
Cadaver upper and lower legs and arms of the same donor
Preparation
Supplies/Equipment needed for experimentation
- Supplies: scalpel, forceps, 16 registration markers, Optotrak sensor (for specimen), Optotrak sensor (for ultrasound), 2 drills, drill bit+tap 8-32 (for registration markers), drill bit (pre-drill for screws), drill bit+tap (for Optotrak sensor plug), ultrasound gel, pens, washable markers, cloth tape measure, wash cloths
- Equipment: Optotrak camera system, Optotrak probe, Ultrasound system with foot-switch, 14L5 probe, 9L4 probe, probe casings, load-transducer computer, load-transducer unit, ATI IFPS box, blue DAQ unit, beige DAQ unit, cadaver fixture
Specimen Preparation Imaging and Indentation
One specimen (e.g. Upper and Lower Leg) will be used at a time
Preparing Ultrasound
Refer to in vivo protocol
Procedures
Antropometric Measurements
Refer to in vivo protocol
Optotrak Marker Assembly on Bones
- Mounting of Optotrak markers:
Leg
- Place the femur Optotrak marker on femur base plug
- Place the tibia Optotrak marker on tibia base plug
Arm
- Place the radius Optotrak marker on radius base plug
- Place the humerus Optotrak marker on humerus base plug
Acquisition of Registration Marker Locations and Anatomical Landmarks
Registration marker data collection
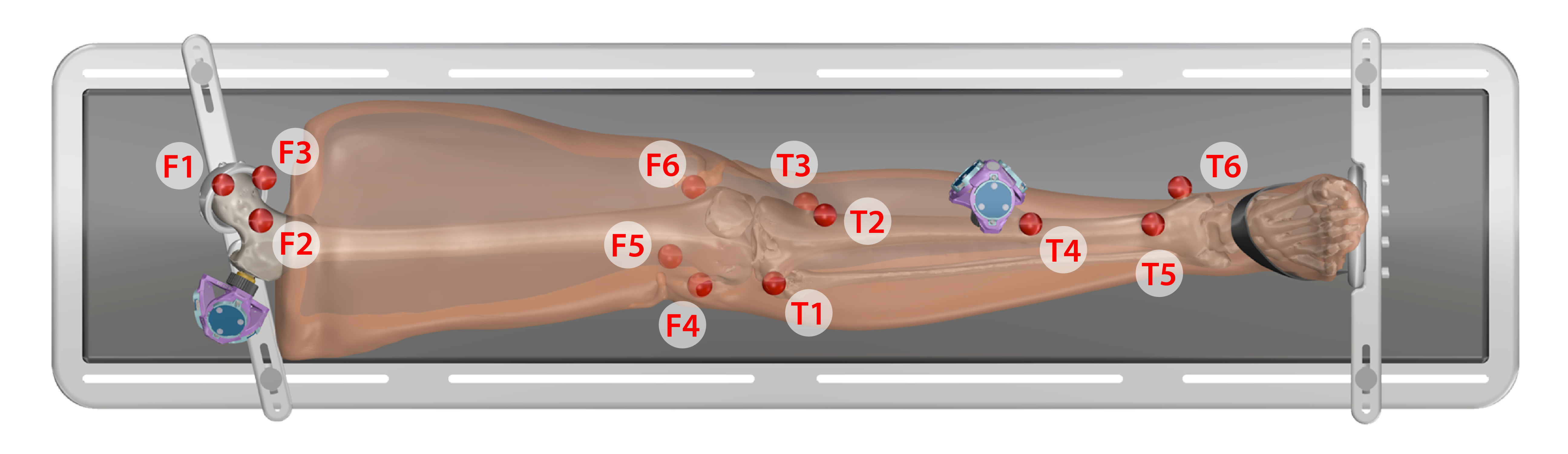
- Keep specimen within Optotrak measurement view.
- Use the digitizing probe to record registration marker locations along with Optotrak marker position/orientation output, measured with respect to the global Optotrak coordinate system for each respective bone
- Ten points on each spherical marker should be digitized such that they are distributed evenly about the sphere surface
- The spheres should be digitized in the following order:
Leg
- Proximal Femur
- F1. Central
- F2. Lateral
- F3. Medial
- Distal Femur
- F4. Lateral epicondyle
- F5. Anterior
- F6. Medial epicondyle
- Proximal Tibia
- T1. Laterial tibial condyle
- T2. Tibial tuberosity
- T3. Medial tibial condyle
- Distal Tibia
- T4. Shaft
- T5. Distal anterior lateral surface
- T6. Medial malleolus
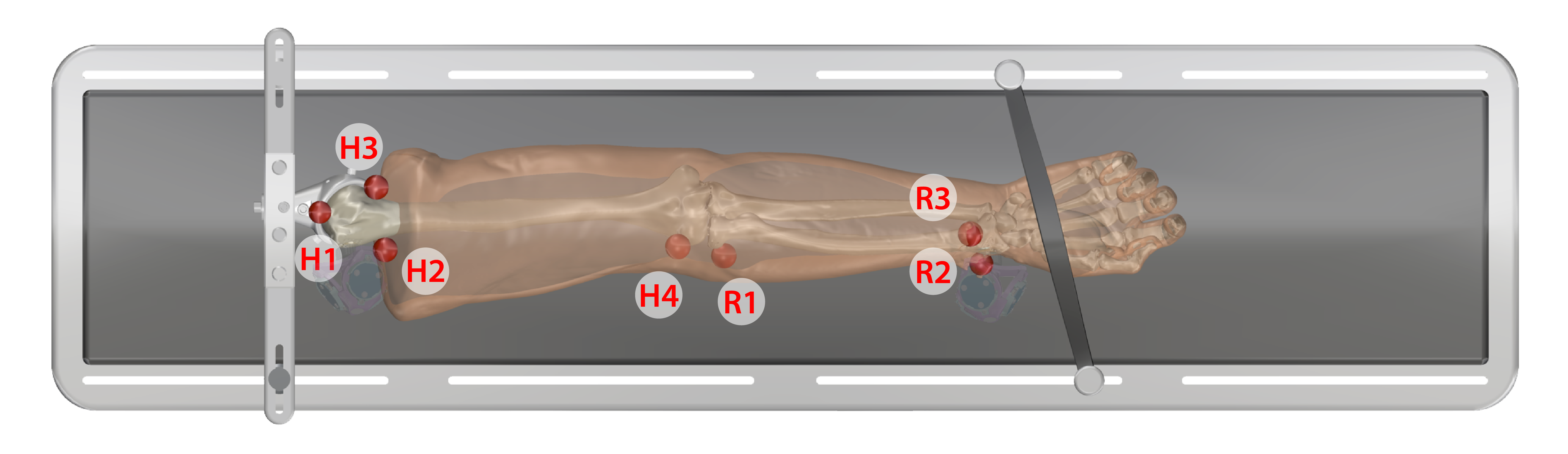
Arm
- Humerus
- H1. Anterior proximal head
- H2. Lateral proximal
- H3. Medial proximal
- H4. Later epicondyle
- Radius
- R1. Neck of radius
- R2. Lateral distal
- R3. Anterior lateral
Anatomical Landmark data collection
- Keep specimen within Optotrak measurement view.
- Use the digitizing probe to record anatomical landmark locations along with Optotrak marker position/orientation output, measured with respect to the global Optotrak coordinate system for each respective bone.
- The following anatomical landmarks will be collected:
Leg
- Femur
- Lateral Femoral Epicondyle
- Medial Femoral Epicondyle
- Femoral Head Point 1
- Femoral Head Point 2
- Femoral Head Point 3
- Femoral Head Point 4
- Tibia
- Lateral Tibial Plateau
- Medial Tibial Plateau
- Lateral Malleolus
- Lateral Malleolus (again)
- Medial Malleolus
- Medial Malleolus (again
Arm
- Humerus
- Lateral Epicondyle
- Medial Epicondyle
- Humeral Head Point
- Ulna
- Lateral Epicondyle
- Medial Epicondyle
- Ulnar Styloid
Note that the specifications dictate that all specimens should be from the right side. If the data collection software is used to collect data from a left specimen, the data file labels will be incorrect as right handed coordinate systems will be created for the left knee. The following chart describes the anatomical meaning for each segment.

Segment Coordinate system definition (Right and Left) |
||||
|
Left |
Right |
Left |
Right |
Ultrasound Indentation
Refer to in vivo wiki as it is the same protocol.
Tissue Harvesting
A region of tissue (skin fat muscle) will be excised from the indentation region of the thigh. This will be labeled and immediately frozen as is. Samples will be cut to size prior to mechanical testing.
Mechanical Testing
Need:
- Skin and Muscle Punch (dumbbell)
- Fat Punch (cylinder)
Preparation
Six equally sized samples will be collected from the indentation region. The region will be excised, then samples will be dissected.
- Skin
- Fat
- Muscle
- Equally sized samples will be collected from the various interfaces
- Skin-fat
- Skin-muscle
- Muscle-muscle
- Fat-muscle (if any)
- 4 samples from each interface will be collected
- Cylindrical shape across the interface
- Strip across interface
- Strip along interface (tensile test)
- Strip along interface (shear test)
Testing
Tensile Testing
- Samples to be tested
- 3 dumbbell skin
- 1 dumbbell muscle
- Strip across interface (4 total)
- Strip along interface (4 total)
Compression (unconfined)
- Samples to be tested
- Cylinder fat
- Cylinder muscle ( + fascia) transverse axis
- Cylinder samples across each interface (4 total)
Failure Tests
- yield, ultimate strength, failure strain each tissue sample
Output
Execution of specifications. see InVitroExperiments